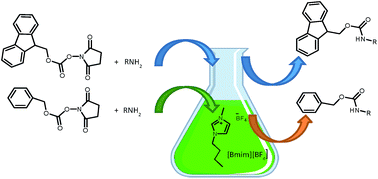
An Efficient Synthesis of Nα-Protected Amino and Peptide Acid Aryl Amides via Iodine-Mediated Oxidative Acylation of Nα-Protected Amino and Peptide Thioacids

Molecules | Free Full-Text | One-Pot and Catalyst-Free Transformation of N- Protected 1-Amino-1-Ethoxyalkylphosphonates into Bisphosphonic Analogs of Protein and Non-Protein α-Amino Acids

Solventless Synthesis of N-Protected Amino Acids in a Ball Mill | ACS Sustainable Chemistry & Engineering
Fmoc-L-Cysteine-(Acetamidomethyl), 5 g, CAS No. 86060-81-3 | Fluorenylmethylene / Fmoc | Amino acids, protected | Amino Acid Derivatives | Amino Acids and Amino Acid Derivatives | Organic & Bioorganic Chemicals | Chemicals | Carl Roth - International

Scheme 1. The stereoselective transformation of N-protected α-amino... | Download Scientific Diagram
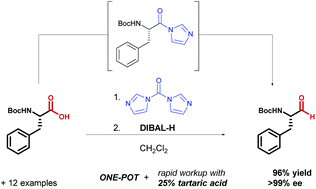
A rapid and efficient one-pot method for the reduction of N-protected α-amino acids to chiral α-amino aldehydes using CDI/DIBAL-H - Organic & Biomolecular Chemistry (RSC Publishing)

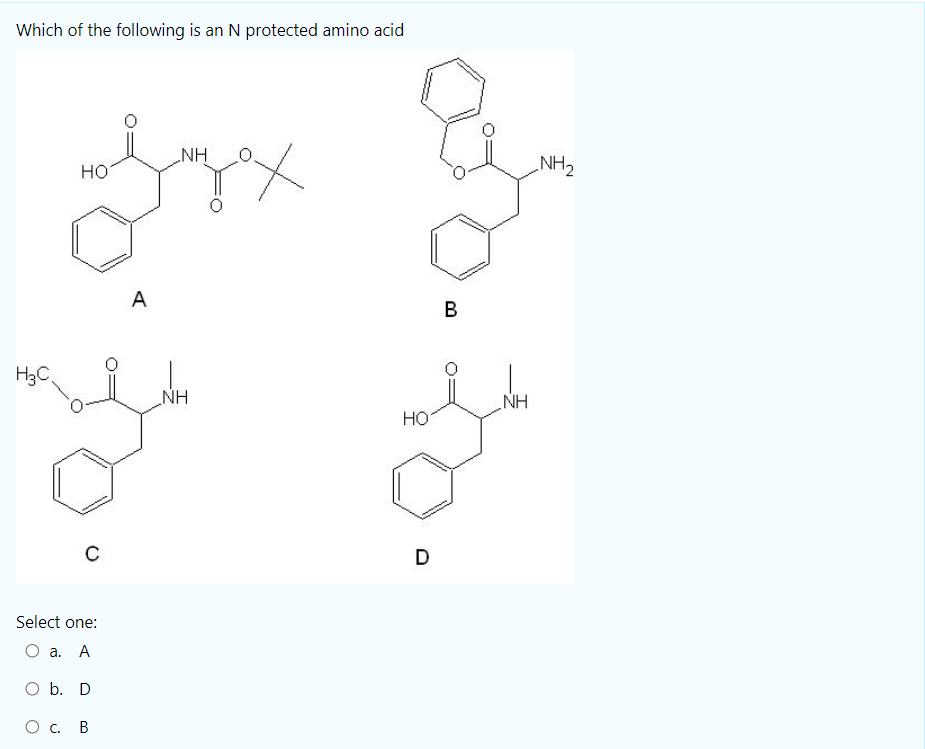
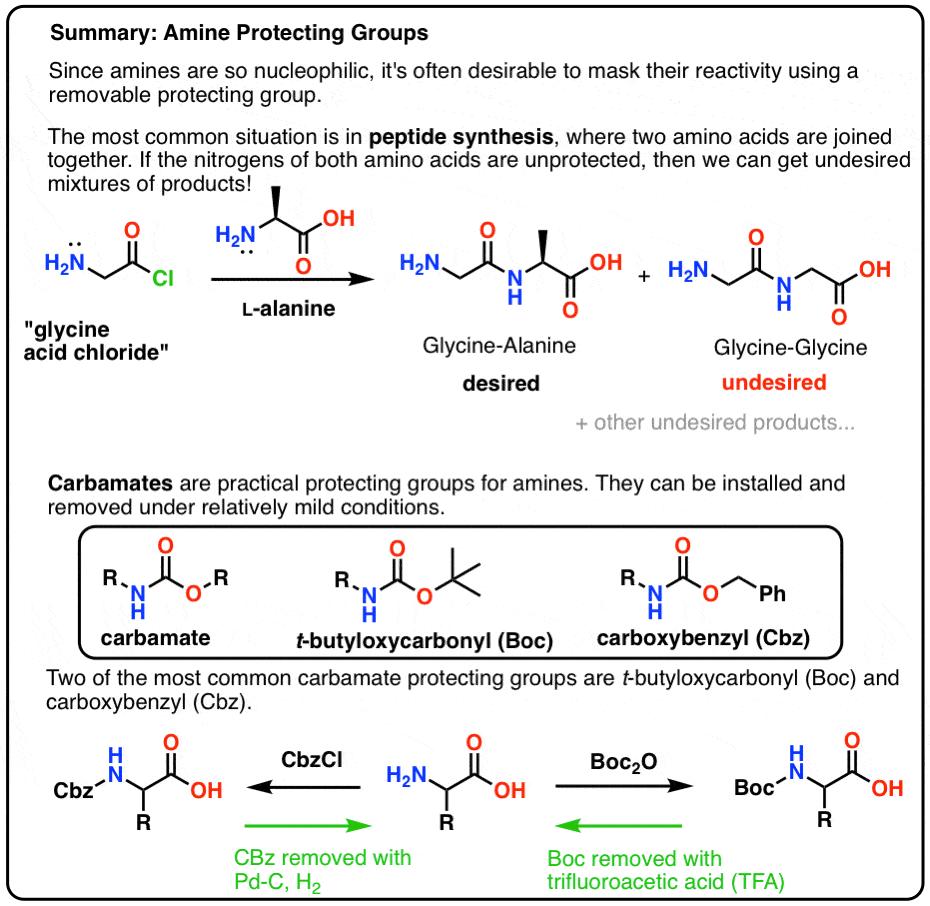
Tertiary-butoxycarbonyl (Boc) – A strategic group for N-protection/deprotection in the synthesis of various natural/unnatural N-unprotected aminoacid cyanomethyl esters - ScienceDirect
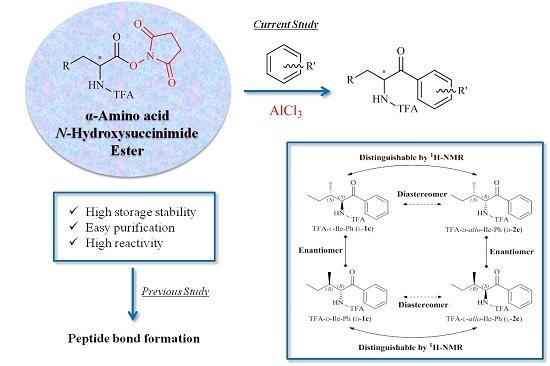
Molecules | Free Full-Text | Synthesis of Chiral TFA-Protected α-Amino Aryl-Ketone Derivatives with Friedel–Crafts Acylation of α-Amino Acid N-Hydroxysuccinimide Ester






![PDF] Solventless Synthesis of N-Protected Amino Acids in a Ball Mill | Semantic Scholar PDF] Solventless Synthesis of N-Protected Amino Acids in a Ball Mill | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/5aa20338b01d225770af7ad1956ad6da9c75ac10/3-Table1-1.png)
![Boc-Amino Acids [N-Protected Amino Acids] | TCI AMERICA Boc-Amino Acids [N-Protected Amino Acids] | TCI AMERICA](https://www.tcichemicals.com/medias/B1186.jpg?context=bWFzdGVyfHJvb3R8Mjk5MTJ8aW1hZ2UvanBlZ3xoOTIvaGZmLzg5Mjg2NDczODEwMjIvQjExODYuanBnfGU3M2ZlN2FjYmM4ZTQ0ZTNmNmE2MGIwZTFkMDIwMzNkYjA5OTc3MDBjZTgzNGYwOGUzZWU3ZWU0NDcxM2Q0MWI)