acid base - What is the relative rate of diffusion of ammonia to hydrogen chloride, both in gaseous states? - Chemistry Stack Exchange

Graph of diffusion constant versus temperature. Solid lines represent... | Download Scientific Diagram

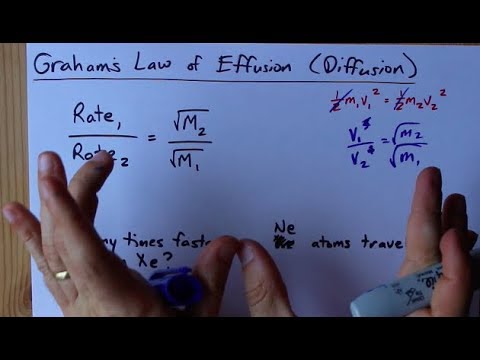
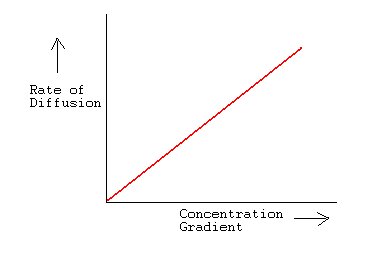
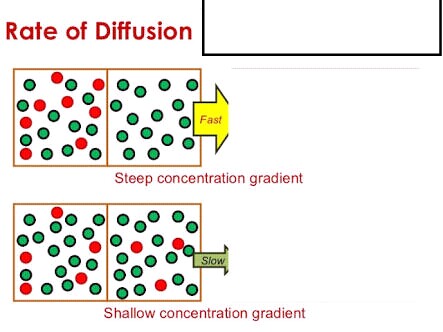
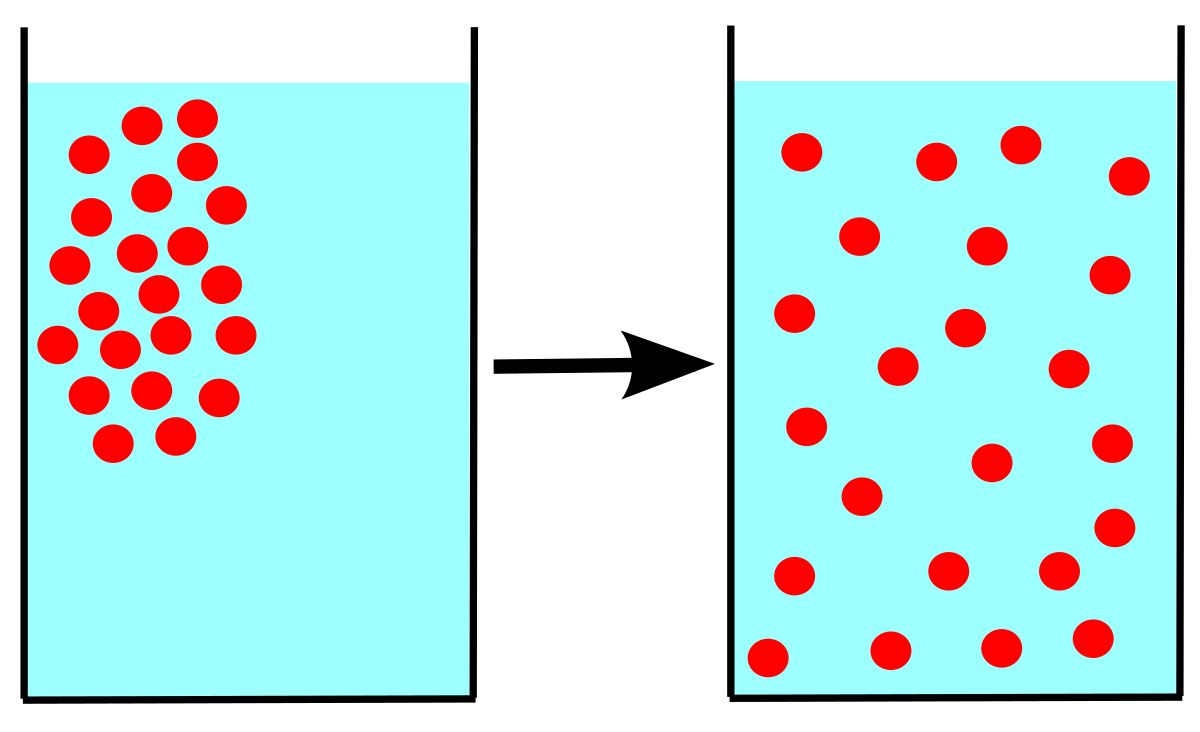
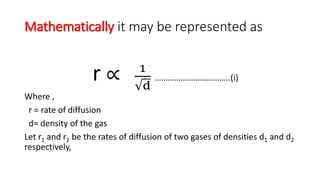
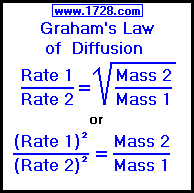
SOLVED:The rate of diffusion of a gas is proportional to: (a) (P)/(√(d)) (b) (P)/(d) (c) √((P)/(d)) (d) (√(P))/(d)